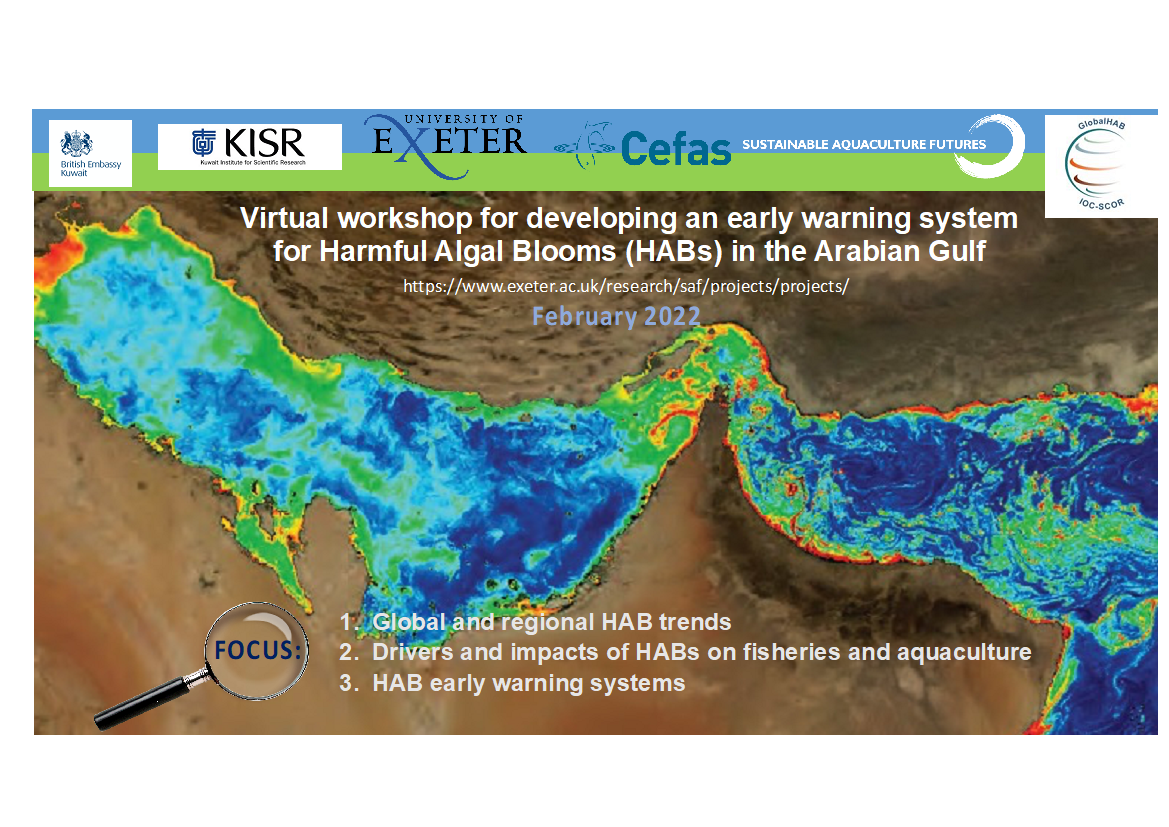
PROJECT TITLE: Workshop-based scoping study for developing an early warning system for Harmful Algal Blooms (HABs) in the Arabian Gulf
Scope: Global with Middle East regional focus
Type: Capacity development and networking
Timeframe: 22 and 23 February 2022
Principal Investigators:
Dr Ross Brown
Address: University of Exeter, Biosciences, Geoffrey Pope Building, Stocker Road, Exeter, EX4 4QD, UK.
Tel: +44 7587702353
E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Dr Qusiae Karam
Address: Crises Management & Decision Support Program, Kuwait Institute for Scientific Research, PO Box 24885, Safat, 13109, Kuwait.
Tel: +965 24989000 ext. 9107/ 9081
Email: This email address is being protected from spambots. You need JavaScript enabled to view it.
Introduction to the workshop
A virtual workshop is planned for February 22-23 2022 (12:00-15:00 UK time) to explore existing knowledge and data on Harmful Algal Blooms (HAB) and impacts on fisheries and finfish aquaculture in the Arabian Gulf. The workshop is being sponsored by the UK and Kuwait Governments and is endorsed by GlobalHAB. The theme of first day is understanding the susceptibility of the Arabian Gulf and adjoining sea areas to harmful algal blooms (HABs) and impacts on fish health and food safety, and the second day would focus on developing early warning systems (EWS) for HABs for mitigating impacts on fisheries and aquaculture.
Background Information
Global aquaculture production from finfish, shellfish and seaweed is projected to grow by 32% from 2018-2030, helping to ensure food security and alleviate pressures on wild stocks from overharvesting and climate change. In the Arabian Gulf finfish aquaculture alone is projected to grow by over 7% between 2018 and 20241. However, the growth of the aquaculture industry is threatened by HABs, which are being observed increasingly at aquaculture sites around the globe, with some HAB species causing mass fish mortalities. Numerous mass fish kills have been recorded in the Arabian Gulf and Sea of Oman and many of these coincide with blooms of ichthyotoxic HAB species and/or high biomass blooms that subsequently decay and cause oxygen depletion in the water column2-4. As an example, in the figure above, chlorophyll concentrations captured by the MODIS Aqua sensor (NASA) represent a bloom of the harmful alga Margalefidinium (Cochlodinium) spp. (in Anderson et al., 2017; Courtesy of R. Kudela and NASA).
References
1) Berdikeeva S. (2019). The Rise of Fish Farming in Over-Fished Gulf Nations. Inside Arabia 27 October 2019. https://insidearabia.com/the-rise-of-fish-farming-in-over-fished-gulf-nations/
2) Al-Alawi A (2018). Harmful Algal Blooms in Oman. Meeting of the Regional Task Force on Eutrophication and HABs Muscat, Sultanate of Oman, 16-18 January 2018. ROPME/WG-176/4/
3) Al-Yamani FY, Polikarpov I, Saburova M (2020): Marine life mortalities and Harmful Algal Blooms in the Northern Arabian Gulf, Aquatic Ecosystem Health & Management. https://doi.org/10.1080/14634988.2020.1798157
4) Attaran-Fariman G (2018). HABs and Phytoplankton Cysts in the ROPME Sea Area. Meeting of the Regional Task Force on Eutrophication and HABs Muscat, Sultanate of Oman, 16-18 January 2018. ROPME/WG-176/4/
Arabian Gulf Harmful Algal Blooms Workshop - programme
DAY 1 (22 February, 12:00-15:00 UK time)
Understanding the susceptibility of the Arabian Gulf and adjoining sea areas
to harmful algal blooms (HABs) and impacts on fish health and food safety
Session chair: Qusaie Karam
1. Welcome & aims of the workshop – Qusaie Karam – Kuwait Institute for Scientific Research (KISR) (5 min)
2. Opening address – Sunny Ahmed (Deputy Ambassador for Kuwait) (5 min)
3. Global perspective on HABs, environmental drivers & impacts – Elisa Berdalet (IOC SCOR GlobalHAB program) (20 min)
4. Regional HAB events and impacts on fisheries/aquaculture in the Regional Organization for the Protection of the Marine Environment (ROPME) Sea Area (RSA):
o Sea of Kuwait – Environmental mechanisms associated with algal blooms in the Arabian Gulf - Yousef Al-Osairi (KISR) (20 min)
o Sea of Oman - Decision and Information System for the Coastal Waters of Oman - Ms. Suad Al-Bemanyah (Ministry of Agricultural Wealth, Fisheries and Water Resources, Oman) (20 min)
5. Break (15 min)
6. The status and trajectory of aquaculture in the Arabian Gulf and Sea of Oman – Patrick White FAO consultant (20 min)
7. Shrimp aquaculture and general aquaculture: an overview, challenges, and accomplishments - Sherain Al-Subaie (KISR) (20 min)
8. Environmental extremes and additional pressures on marine ecosystems in the RSA – Michelle Devlin (CEFAS) (20 min)
9. Linking environmental factors, HAB events and impacts on fish and shellfish - Adam Lewis (CEFAS), Ross Brown (University of Exeter) (20 min)
10. Discussion on sampling strategies, tools and data availability, integration and analysis (15 min)
DAY 2 (23 February, 12:00-15:00 UK time)
Developing early warning systems (EWS) for HABs for mitigating impacts on fisheries and aquaculture
Session chair: Ross Brown
11. Introduction and recap (from Day 1) on aims of the workshop – Ross Brown (University of Exeter) (5 min)
12. Understanding impacts of HABs on fish farms and vice versa based on lessons learned in:
o Chile - Jorge Mardones (CREAN) (15 min)
o Control of HABs in China using a modified clay approach - Isaac Yongquan Yuan (CAS) (15 min)
13. Existing HAB observation and EWS in the RSA
o Kuwait Territorial Waters - EWS for HABs and fish kill Incidents - Qusaie Karam (KISR) (15 min)
o Eastern Gulf and Sea of Oman - Gilan Attaran-Fariman (Chabahar Maritime University, Iran) (15 min)
14. Break (15 min)
15. Other observation and EWS for HABs o HAB Early Warning Systems in Chile - Alejandro Clément (Plancton Andino, Chile) (15 min)
o HAB Early Warning Systems in North America - Raphe Kudela (University of S California) (15 min)
o Changes and complexity of HABs in Asia - Patricia Glibert (University of Maryland) (15 min)
o HAB Early Warning Systems in the UK - Keith Davidson (SAMS) (15 min)
16. Break (15 min)
17. Discussion on next steps for further developing a HAB EWS for the RSA (30 min)
o What are key data/ knowledge gaps and research priorities?
o Data linking HAB occurrence and fish kills?
o Funding opportunities?
18. Sum up and close workshop (Ross Brown, Elisa Berdalet Po Teen Lim) (10 min)
Gulf HAB workshop videos and proceedings:
Part 1 https://www.youtube.com/watch?v=1oYVQYMmMhc
Part 2 https://www.youtube.com/watch?v=PWvROnKbmDE
Part 3 https://www.youtube.com/watch?v=JkzfTmWM3oE
Part 4 https://www.youtube.com/watch?v=BgwTKTv9nHI
Part 5 https://www.youtube.com/watch?v=uNkt8ANJ95g
Part 6 https://www.youtube.com/watch?v=CPXjVn_abBY
Gulf HAB workshop proceedings.pdf
PROJECT TITLE: Citizen Science Program in the Strait of Georgia, Canada
Scope: Regional
Type: Monitoring
Timeframe: January 2015 to December 2021
Program manager: Dr. Isobel Pearsall
Address: Pacific Salmon Foundation, 1682-300 West 7th Avenue, Vancouver, BC, V6J 4S6, Canada
E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Home page URL:
https://marinesurvivalproject.com/research_activity/list/citizen-science-program/
http://sogdatacentre.ca/people/citizen-science/
https://www.facebook.com/CitizenSciencePhytoplankton/
Other key people:
Nicole Frederickson (program coordinator)
Svetlana Esenkulova (harmful algae component)
Objectives:
The goal of the Citizen Science Program in the Strait of Georgia is to collect and disseminate detailed oceanographic data. This program was implemented by the Pacific Salmon Foundation (PSF) with support from the Oceans and Networks Canada (ONC) and Department of Fisheries and Oceans (DFO). The program has been operating since 2015, and was endorsed by the GlobalHAB program in January 2020. Trained members of local communities (referred to as “citizen scientists”) collect information at ~55 defined locations (Fig. 1) on, or as close to, the same day approximately every two weeks between February and October, annually between 2015-2021. At each station, CTDs (conductivity, temperature, depth) are deployed and water samples are taken for nutrient analysis and phytoplankton taxonomy. The scope and coverage of this program are unprecedented; more details about this program and the CitSci dataset, as well as figures showing oceanographic conditions over 2015-2019, are provided in Pawlowicz et al. (2020). Data are stored at http://sogdatacentre.ca/
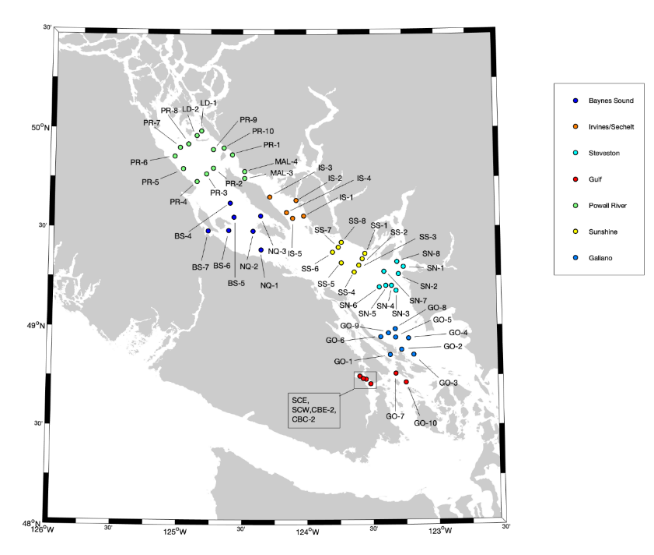
Figure. 1 Map of the Strait of Georgia with CitSci program sampling locations in 2020.
Different colours represent different patrols.
During six years of observations to date, heavy blooms of potentially harmful blooms were caused by Noctiluca scintillans (Fig. 2, 3), Heterosigma akashiwo, Pseudo-nitzschia spp., Rhizosolenia setigera, Gonyaulax spp., and Dictyocha spp. Investigation of the relationship between environmental factors and bloom dynamics is underway.

Figure. 2 Noctiluca scintillans bloom, Salt Spring Island, May, 2 2018. Photo by Michael Bahrey.

Figure. 3 Cell of Noctiluca scintillans with Chaetoceros spp. in food vacuoles,
Citizen Science sample. Photo by Svetlana Esenkulova
Outcomes:
Pawlowicz, R., Suzuki, T., Chappel, R., and Esenkulova, S. 2020. Atlas of Oceanographic Conditions in the Strait of Georgia (2015–2019) based on the Pacific Salmon Foundation’s Citizen Science Dataset. Can. Tec. Rep. Hydrogr. Ocean Sci. 3374: vii + 116 p.
Esenkulova, S., Suchy, K., Pawlowicz, R., Costa, M., & Pearsall, I. Harmful algae and oceanographic conditions in the Strait of Georgia, Canada based on citizen science monitoring. Frontiers in Marine Science,1193.
Annual reports of the harmful algae observations are published in the State of the Pacific Ocean:
- 2019 - Esenkulova, S., Frederickson, N., Pearsall, I. Harmful algal blooms in the Salish Sea. https://waves-vagues.dfo-mpo.gc.ca/Library/40884569.pdf
- 2018 - Esenkulova, S., Pearsall, I. Harmful algal blooms in the Salish Sea. https://waves-vagues.dfo-mpo.gc.ca/Library/4081306x.pdf
- 2017 - Esenkulova, S. Pawlowicz, R., Pearsall, I. Nutrients, the phytoplankton community and harmful algae in the Salish Sea. http://waves-vagues.dfo-mpo.gc.ca/Library/40717914.pdf
- 2016 - Esenkulova, S., Pearsall, I. The phytoplankton community in the Salish Sea. http://waves-vagues.dfo-mpo.gc.ca/Library/40617944.pdf
Several reports were published in the Harmful Algae News:
- Esenkulova, S., Pearsall, I., 2019: Citizen Science oceanography in the Strait of Georgia, Canada – an overview of five years operations. Harmful Algae News 63, 12-13. http://www.e-pages.dk/ku/1439
- Ecology of Alexandrium spp. in the Strait of Georgia, British Columbia, Canada 2015. Esenkulova, Pearsall, Novak, 2017: Harmful Algae News 56, 7-8. http://www.e-pages.dk/ku/1276/
- Observations of Heterosigma akashiwo bloom and associated wild salmon lethargic behavior in Cowichan Bay, Canada, 2014. Esenkulova, Luinenburg, Neville, Trudel, 2014. Harmful Algae News 50, 16-18. http://www.e-pages.dk/ku/1086/